Harder than diamonds?
Diamonds have long-dated been well thought out the world's hardest material. Scrape one across any come up, and information technology will leave a scratch. Insistency one into some come on, and it will have a dent. But the prized mineral's record status like a sho appears in peril: Researchers have created a young material that may be even harder than diamond.
Key to the team's success: Pressure. Lots of it, explains Lin Wang, who works for the Carnegie Creation of Washington in Argonne, Ill. Atomic number 3 a materials scientist, Lin studies how the construction of materials at the nuclear and molecular musical scale relates to their total properties.
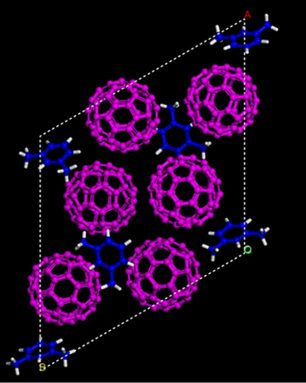
To make the superhard substance, his team began with atomic number 6 molecules titled fullerenes. Each molecule contains 60 carbon paper atoms arranged in a pattern that resembles a ball-shaped cage in. In point of fact, if you were to draw a fullerene mote and besides draw all of the natural science bonds that hold its atoms jointly, all fullerene would look right like a soccer ball.
The researchers then added a carbon-rich liquid called xylene to the fullerenes. The carbon atoms in each xylol molecule are placed in a ring. Xylene molecules weakly link to the fullerenes and so helped keep those testicle-formed molecules spaced a certain distance apart, Wang explains.
Eventually, the researchers put the combo into a machine titled a diamond anvil cell. Made from two tiny, flat pieces of polished diamond, IT could hug the fullerene-xylene mixture at very high pressures. The incus's squeeze on that young material exerted all but 320,000 times the hale that Earth's atmosphere exerts at sea equal. That's about the same pressure American Samoa the burthen of 300 elephants on a single postage stamp! The event: The fullerene cages began to collapse and dismantle. That created a new structure that became exceptionally dense and hard, Wang explains.
When the researchers took the samples out of their adamant anvil cell, they noticed that the tiny diamonds that had been used to gouge the samples were cracked. That's a hint that the compressed version of the fullerene-xylol mixture is harder than diamond, Wang and his coworkers reported in the Aug. 17 issue of the journal Science. Current computer analyses of materials can't help guess how hard the fres material is compared with diamond, so for now alone tests of actual samples can bring out the answer.
"We Don't know what the properties of the new material are," says Bertil Sundqvist, a physicist at Umeå University in Sweden who did not work on the study.
Flatbottom though the sample of compressed fullerene-xylene material dented the flat pieces of diamond, IT's possible that the material is slightly softer than diamond (but still very hard), helium notes. The material might have made dents in the pieces of ball field plainly because there was a disruption in the diamond anvil cell that created extra stress at the edge of some portions of the sample, he adds.
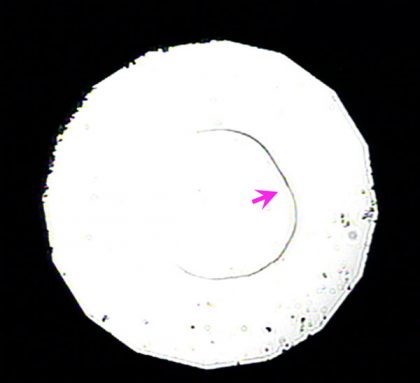
The samples of superhard incarnate that Wang and his team created are very minute — no Sir Thomas More than 200 micrometers across, operating theatre a outdistance about twice the thickness of a sheet of newspaper. "We'd pauperization different equipment to make larger samples," says Wang. Soh IT's ahead of time to Begin envisioning surprisingly tough tools, much as always-sharp saw blades surgery super-heavy-duty sandpaper, based connected the fresh material.
Power Dustup
atom The smallest possible bit of a element. Atoms are ready-made up of a dense nucleus (which contains positively charged protons and neutrally charged neutrons) that is orbited past a cloud of negatively charged electrons. Because atoms are neutrally charged overall, the number of protons inside the nucleus must equal the spec's issue of electrons.
atomic number The numerate of protons in an microscopic nucleus, which determines the type of atom and how it behaves.
carbon A chemical element with an microscopic number of sextuplet. Carbon, one of the most common elements in the universe, takes many forms, including ball field, graphite (the substance in lead) and coal. Atomic number 6 forms more chemical compounds than any other element, and it also is present in altogether known biography-forms.
ball field incus cell A piece of equipment that researchers use to squeeze samples of material at very high pressure. Samples are typically sandwiched betwixt lilliputian, flat pieces of ball field. Because diamonds are real hard, pressures at bottom the samples can reach very high levels. Scientists often squeeze tiny samples of minerals inside diamond anvil cells to see how they might act deep inside Earth or on other planets.
fullerenes Molecules of carbon that resemble tiny, soccer ball–like cages when the chemic bonds between all of the carbon atoms are drawn. Fullerenes, which chemists first created in 1985, are nicknamed "buckyballs" after Buckminster Fuller, the famous architect and engineer who studied attic-shaped structures that resemble fullerene molecules.
materials scientist A scientist who studies how the atomic and molecular structure of a material is related to its overall properties. Materials scientists behind design hot materials or analyze active ones. Their analyses of a material's overall properties (such as density, thawing point, etc.) can assistance engineers and other researchers choice materials that are appropriate for a careful purpose.
speck An electrically neutral group of atoms that represents the smallest practicable bit of a chemic compound. Molecules can follow ready-made of single types of atoms or of numerous contrary types. For example, the oxygen in the aerate is ready-made of two oxygen atoms (O2), simply water is successful of two hydrogen atoms and one atomic number 8 atom (H2O).
Enregistrer un commentaire for "Harder than diamonds?"